Mitochondrial Dysfunction: How Your Cell Powerhouses Influence Chronic Disease
Introduction to Mitochondrial Dysfunction
Mitochondria are often called the "powerhouses" of the cell, but their function goes far beyond producing energy. These critical organelles regulate cellular metabolism, survival, and immune defense. When mitochondria become dysfunctional, the consequences affect the entire body.
This article explores what mitochondria do, what mitochondrial dysfunction is, how viral infections, chronic inflammation, and gut dysbiosis cause it, and ways to prevent or reverse mitochondrial damage.
What Are Mitochondria?
Mitochondria are double-membraned organelles found in almost all the cells that have the nucleus (a structure in the cell that contains the DNA of the organism) (Nunnari & Suomalainen, 2012). They contain their own DNA, inherited maternally, and produce most of the cell’s energy unit called the ATP (Gray, 2012).
Besides energy production, mitochondria control how a cell dies (called apoptosis ), how it stores calcium and how it signals the immune system (Spinelli & Haigis, 2018; Rizzuto et al., 2012).
Key Functions of Mitochondria
Understanding mitochondrial functions helps explain why mitochondrial dysfunction can have widespread effects. Their primary roles include:
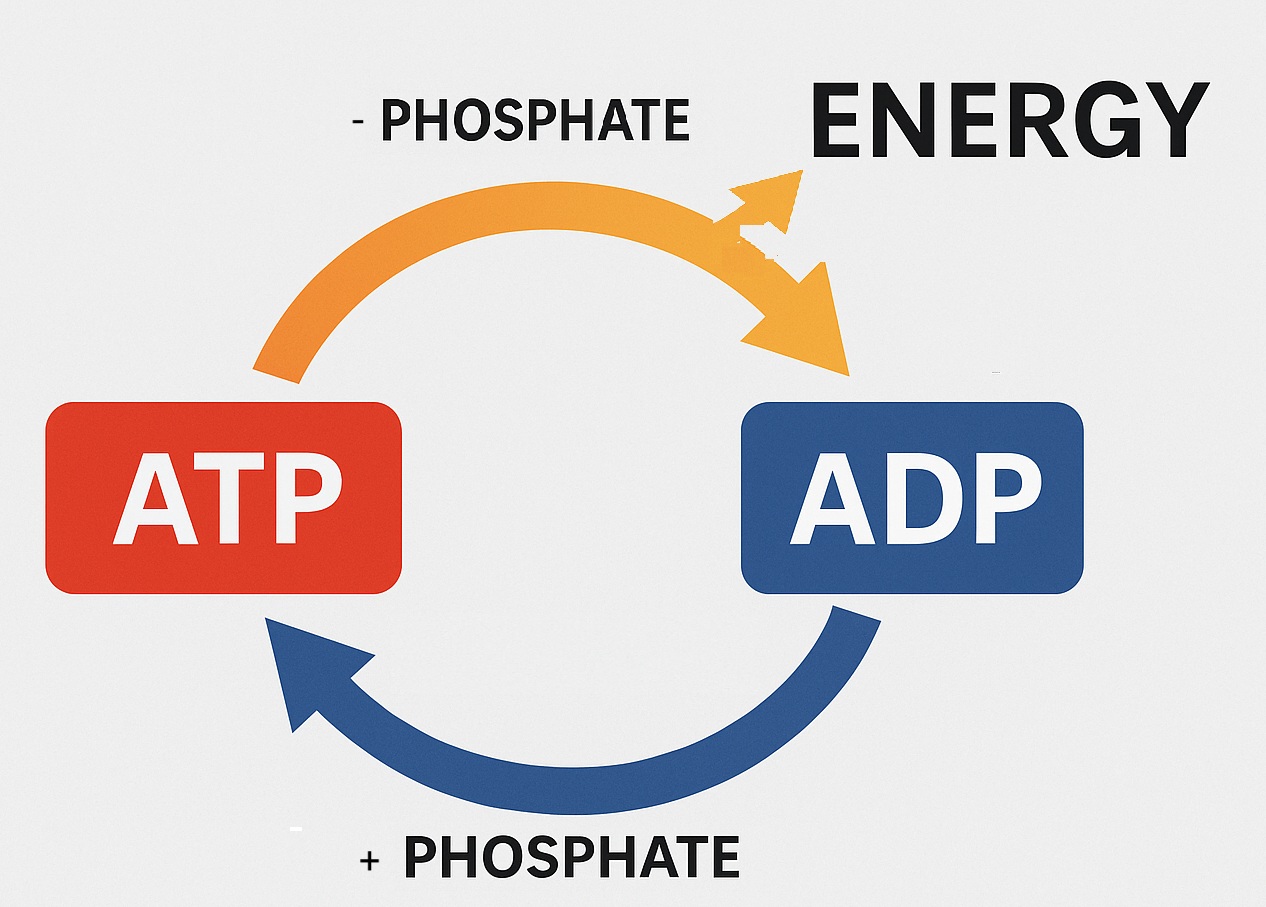
• Energy Production
It puts the phosphate particle back on the ADP (adenosine diphosphate) to create ATP (adenosine triphosphate). (Spinelli & Haigis, 2018).
This diagram shows how this happens:
• Apoptosis Regulation
Mitochondria tells the cell that it is time to die. This is needed so that new cells can be regenerated. (Tait & Green, 2012).
• Reactive Oxygen Species (ROS) Management:
Mitochondria produces these unstable oxygen particles that when released especially in infection, help to fight the infections. (Shadel & Horvath, 2015).
• Calcium Homeostasis:
Calcium is needed by our cells to communicate with each other and also to help our muscles contract. Mitochondria has a supporting role in this process. (Rizzuto et al., 2012).
• Immune Modulation:
Mitochondria is the first responder of the immune system that works as soon as infection occurs to try to stop the infection. (West et al., 2011). When the body picks up that there is a virus/bacteria or invasion, the mitochondria releases a whole lot of unstable oxygen particles, that hopefully can disrupt and kill these invaders. It takes the normal immune system (white cells, lymphocytes etc) around 3 days to 5 days before it is ready to take over the body's defense fully. In the meantime, during these 3 days to 5 days, the mitochondria works hard to be the first responder. But after the normal defense system takes over, the mitochondria hopefully reverts back to its energy production role.
Any disruption of these processes can contribute to disease.

What Is Mitochondrial Dysfunction?
Mitochondrial dysfunction occurs when mitochondria fail to perform their roles properly. This can lead to reduced ATP production, excessive ROS production, impaired calcium balance, and mitochondrial DNA (mtDNA) mutations (Yong et al., 2020).
Mitochondrial dysfunction is central to many diseases, including diabetes, Alzheimer’s disease, cardiovascular disease, and even cancer (Wallace, 2012).
Main Causes of Mitochondrial Dysfunction
Several external and internal factors disrupt mitochondrial health. Understanding these helps in developing preventive strategies.
Viral Infections
Certain viruses directly attack mitochondria, disrupting their function to evade immune detection. For example, hepatitis C virus and HIV can cause the mitochondria to produce a lot more reactive oxygen particles (called free radicals) that the cell can use and therefore leads to the mitochondria getting damaged by its own reactive oxygen particles (West & Shadel, 2017).
Mitochondria has its own system to signal to the rest of the immune system to say there is a virus in the body and it needs more specialized help to deal with it (called the mitochondrial antiviral signaling system (MAVs). Viruses also suppress MAV system, weakening immune responses (Koshiba, 2013).
Chronic Inflammation
Chemicals released during inflammation, like TNF-α and IL-6, damage the mitochondria’s ability to make energy and cause the buildup of harmful free radicals (Zhang et al., 2013).
This oxidative environment leads to a vicious cycle of inflammation and further mitochondrial damage, contributing to diseases such as arthritis and inflammatory bowel disease (Angajala et al., 2018).
Gut Dysbiosis
The gut microbiome influences mitochondrial health profoundly. Gut dysbiosis:
• makes the gut lining "leaky," allowing harmful substances like LPS (which are released by the bacteria when it is being broken down by the immune system or treatments) to enter the bloodstream, which then stresses the mitochondria and causes more damaging free radicals to form. (Bailey et al., 2011).
• reduces production of short-chain fatty acids essential for mitochondrial energy (Donia & Fischbach, 2015).
• increases overall inflammation in the body, harming mitochondria (Rooks & Garrett, 2016).
Maintaining gut health is crucial for preventing mitochondrial dysfunction.
How Mitochondrial Dysfunction Leads to Chronic Disease
Mitochondrial dysfunction is a key driver in many chronic conditions:
Neurodegenerative Diseases
In diseases like Alzheimer’s, Parkinson’s, and ALS, damage to the mitochondria happens early on and speeds up the loss of brain cells. (Lin & Beal, 2006; Swerdlow, 2018).
Metabolic Disorders
When mitochondria can’t properly burn fat for energy, it can lead to problems like insulin resistance, type 2 diabetes, and weight gain. (Lowell & Shulman, 2005; Petersen et al., 2004).
Cardiovascular Disease
When mitochondria don’t work properly, the heart doesn't get enough energy, which can cause irregular heartbeats and damage blood vessels, leading to clogged arteries. (Madamanchi & Runge, 2007; Murphy et al., 2016).
Cancer
Cancer cells take advantage of how mitochondria produce energy, helping them grow quickly, and they often have damaged mitochondrial DNA and high levels of stress inside the cell. (Vyas et al., 2016; Reznik et al., 2017).
How to Prevent or Reverse Mitochondrial Dysfunction
Lifestyle strategies and targeted therapies can enhance mitochondrial health.
Nutrition for Mitochondrial Support
• Antioxidant-Rich Diet: Foods like berries and greens combat ROS (Pisoschi & Pop, 2015).
• Mitochondrial Nutrients: Coenzyme Q10, magnesium, B vitamins, and alpha-lipoic acid support ATP production (Smith & Hagen, 2003).
• Polyphenols: Compounds like resveratrol stimulate the cells to create more mitochondria (Baur et al., 2006).
As with every article presented by Maximised Nutrition, it is important to talk to a qualified health professional regarding the right supplement and dose for you, especially if you are on any other pharmaceutical medications.
Exercise
Aerobic and resistance exercise stimulate mitochondrial formation and improve energy efficiency (Hood et al., 2019).
Regular physical activity reduces inflammation, helps the body make more new, healthy mitochondria, and delays mitochondrial aging (Holloszy, 1967).
Fasting and Ketogenic Diets
Intermittent fasting and ketogenic diets train the body to burn fat for energy instead of sugar, which helps the mitochondria work better. (Newman & Verdin, 2014; Veech, 2004).
Ketones also generate less oxidative stress than glucose metabolism.
However, Maximised Nutrition does not recommend ketogenic diet for everyone, especially not to women.
Thus talk to a Clinical Dietitian-Nutritionist before starting a ketogenic diet.
Maximised Nutrition has developed a modified Mediterranean diet strategy that seems to work well for clients. To get an individualised diet plan, contact Maximised Nutrition.
Managing Infections and Inflammation
Treating infections quickly and following habits that reduce inflammation help keep your mitochondria healthy and working properly. (Calder, 2017).
Managing stress, eating foods that fight inflammation, and getting enough sleep help keep your mitochondria strong and healthy.
Supporting Gut Health
Maintaining a balanced microbiome through:
• Probiotics
• Prebiotic fibers
• Limiting unnecessary antibiotics
helps protect mitochondrial function (Zmora et al., 2019).
Conclusion: Protect Your Mitochondria to Protect Your Health
Problems with mitochondria often happen quietly but can lead to many long-term health issues. Knowing what harms them—like viruses, ongoing inflammation, and an unhealthy gut—gives you the power to protect yourself.
By eating the right foods, staying active, trying safe fasting methods, and keeping your gut healthy, you can strengthen your mitochondria and boost your energy and health for years to come.
Start today—nourish your mitochondria and transform your health from the inside out.
References
• Bailey, M. T., Dowd, S. E., Parry, N. M., Galley, J. D., Schauer, D. B., & Lyte, M. (2011). Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infection and Immunity, 79(4), 1509-1519.
• Baur, J. A., Pearson, K. J., Price, N. L., Jamieson, H. A., Lerin, C., Kalra, A., ... & Sinclair, D. A. (2006). Resveratrol improves health and survival of mice on a high-calorie diet. Nature, 444(7117), 337-342.
• Calder, P. C. (2017). Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochemical Society Transactions, 45(5), 1105-1115.
• Donia, M. S., & Fischbach, M. A. (2015). Small molecules from the human microbiota. Science, 349(6246), 1254766.
• Gray, M. W. (2012). Mitochondrial evolution. Cold Spring Harbor Perspectives in Biology, 4(9), a011403.
• Holloszy, J. O. (1967). Biochemical adaptations in muscle. Journal of Biological Chemistry, 242(9), 2278-2282.
• Hood, D. A., Memme, J. M., Oliveira, A. N., & Triolo, M. (2019). Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annual Review of Physiology, 81, 19-41.
• Koshiba, T. (2013). Mitochondrial-mediated antiviral immunity. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1833(1), 225-232.
• Lin, M. T., & Beal, M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature, 443(7113), 787-795.
• Lowell, B. B., & Shulman, G. I. (2005). Mitochondrial dysfunction and type 2 diabetes. Science, 307(5708), 384-387.
• Madamanchi, N. R., & Runge, M. S. (2007). Mitochondrial dysfunction in atherosclerosis. Circulation Research, 100(4), 460-473.
• Murphy, M. P., & Hartley, R. C. (2018). Mitochondria as a therapeutic target for common pathologies. Nature Reviews Drug Discovery, 17(12), 865-886.
• Newman, J. C., & Verdin, E. (2014). Ketone bodies as signaling metabolites. Trends in Endocrinology & Metabolism, 25(1), 42-52.
• Nicholls, D. G., & Ferguson, S. J. (2013). Bioenergetics. Academic Press.
• Nunnari, J., & Suomalainen, A. (2012). Mitochondria: in sickness and in health. Cell, 148(6), 1145-1159.
• Petersen, K. F., Dufour, S., Befroy, D., Garcia, R., & Shulman, G. I. (2004). Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. New England Journal of Medicine, 350(7), 664-671.
• Pisoschi, A. M., & Pop, A. (2015). The role of antioxidants in the chemistry of oxidative stress: A review. European Journal of Medicinal Chemistry, 97, 55-74.
• Reznik, E., Miller, M. L., Şenbabaoğlu, Y., Riaz, N., Sarungbam, J., Tickoo, S. K., ... & Sander, C. (2016). Mitochondrial DNA copy number variation across human cancers. eLife, 5, e10769.
• Rizzuto, R., De Stefani, D., Raffaello, A., & Mammucari, C. (2012). Mitochondria as sensors and regulators of calcium signaling. Nature Reviews Molecular Cell Biology, 13(9), 566-578.
• Rooks, M. G., & Garrett, W. S. (2016). Gut microbiota, metabolites and host immunity. Nature Reviews Immunology, 16(6), 341-352.
• Shadel, G. S., & Horvath, T. L. (2015). Mitochondrial ROS signaling in organismal homeostasis. Cell, 163(3), 560-569.
• Spinelli, J. B., & Haigis, M. C. (2018). The multifaceted contributions of mitochondria to cellular metabolism. Nature Cell Biology, 20(7), 745-754.
• Swerdlow, R. H. (2018). Mitochondria and mitochondrial cascades in Alzheimer's disease. Journal of Alzheimer's Disease, 62(3), 1403-1416.
• Tait, S. W., & Green, D. R. (2012). Mitochondria and cell death: outer membrane permeabilization and beyond. Nature Reviews Molecular Cell Biology, 11(9), 621-632.
• Veech, R. L. (2004). The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins, Leukotrienes and Essential Fatty Acids, 70(3), 309-319.
• Vyas, S., Zaganjor, E., & Haigis, M. C. (2016). Mitochondria and cancer. Cell, 166(3), 555-566.
• Wallace, D. C. (2012). Mitochondria and cancer. Nature Reviews Cancer, 12(10), 685-698.
• Weinberg, S. E., Sena, L. A., & Chandel, N. S. (2015). Mitochondria in the regulation of innate and adaptive immunity. Immunity, 42(3), 406-417.
• West, A. P., & Shadel, G. S. (2017). Mitochondrial DNA in innate immune responses and inflammatory pathology. Nature Reviews Immunology, 17(6), 363-375.
• West, A. P., Shadel, G. S., & Ghosh, S. (2011). Mitochondria in innate immune responses. Nature Reviews Immunology, 11(6), 389-402.
• Yong, J., Kim, J. H., Kim, J., Ryu, S. H., & Suh, P. G. (2020). Mitochondria in cancer metabolism: from physiology to therapy. Biochemical Society Transactions, 48(4), 1197-1206.
• Zmora, N., Suez, J., & Elinav, E. (2019). You are what you eat: diet, health and the gut microbiota. Nature Reviews Gastroenterology & Hepatology, 16(1), 35-56.