Is Chronic Inflammation Silently Harming Your Health? Click to do this 60 second test to find out if it is

GUT DYSBIOSIS AND HUMAN HEALTH
GUT DYSBIOSIS AND HUMAN HEALTH: CAUSES, SYMPTOMS, AND SOLUTIONS
Humans as Metaorganisms: The Holobiont Perspective
In recent years, science has increasingly recognized that humans are not solitary beings but metaorganisms, or holobionts—complex entities composed of the human host and trillions of microbial symbionts that reside within and on us (Bordenstein & Theis, 2015). This intimate relationship, especially in the gut (with the gut microbiota balance), plays a pivotal role in our well-being, making the connection between gut dysbiosis and human health a critical area of research and concern.
Human as "Holobiont" (Copyright Maximised Nutrition)
Our gut microbiota—a dynamic ecosystem of bacteria, archaea, viruses, and fungi—acts as a metabolic organ. When in balance, it supports nutrient synthesis, immune modulation, and pathogen defense. However, when this balance is disrupted, a condition known as gut dysbiosis arises, leading to various health consequences.
What is Gut Dysbiosis?
Gut dysbiosis refers to an imbalance in the composition and function of gut microbiota. This state is characterized by a reduction in microbial diversity, a decrease in beneficial bacteria, and an overgrowth of harmful microbes (Petersen & Round, 2014). Dysbiosis disrupts the symbiotic relationship between host and microbiota, impairing essential biological functions and increasing the risk of disease.
Understanding the link between gut dysbiosis and human health begins with recognizing how microbial imbalance affects basic bodily functions.
What Causes Gut Dysbiosis?
Gut dysbiosis can result from several internal and external factors, many of which are influenced by modern lifestyles:
1. Dietary Factors
High intake of processed foods, sugars, and saturated fats can diminish beneficial bacteria and promote pathogenic strains (David et al., 2014). Low fiber diets reduce substrates for short-chain fatty acid (SCFA)-producing microbes.
2. Antibiotic Overuse
Antibiotics disrupt microbial communities, often killing both harmful and beneficial bacteria. Repeated or long-term use is a leading cause of dysbiosis (Jernberg et al., 2010).
3. Chronic Stress
Psychological stress affects gut motility, immune response, and microbiota composition via the gut-brain axis (Foster et al., 2017).
4. Environmental Toxins
Exposure to pesticides, heavy metals, and industrial chemicals may alter microbial ecosystems and increase intestinal permeability (Kozik et al., 2019).
5. Cesarean Delivery and Lack of Breastfeeding
Early microbial colonization differs in cesarean-born and formula-fed infants, increasing dysbiosis risks later in life (Dominguez-Bello et al., 2010).
Lifestyle and environmental factors significantly influence gut dysbiosis and human health, especially through diet and medication.
Impacts of Gut Dysbiosis and Human Health
The connection between gut dysbiosis and human health extends far beyond digestion. Dysbiosis can influence multiple bodily systems, contributing to chronic diseases, mental health disorders, immune dysfunction, and more.
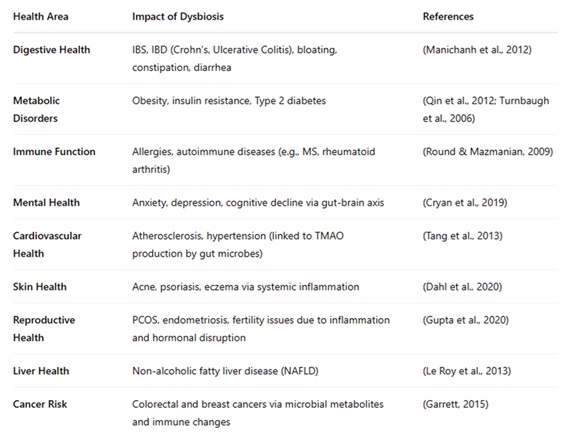
Research continues to unveil how deeply connected gut dysbiosis and human health truly are, with effects reaching far beyond digestion.
Symptoms of Gut Dysbiosis
Dysbiosis often presents with a range of subtle or chronic symptoms. These diverse symptoms reflect the systemic nature of the relationship between gut dysbiosis and human health.
Common signs include:
- Chronic bloating or gas
- Constipation or diarrhea
- Sugar cravings
- Persistent fatigue
- Brain fog or mood swings
- Food intolerances
- Frequent colds or infections
- Skin breakouts or rashes
- Bad breath
- Unexplained weight changes
These symptoms are often overlooked but may signal underlying microbial imbalance (Zmora et al., 2019).
Five Things You Can Do About Gut Dysbiosis
Taking action to restore microbial balance is a powerful step toward improving your health. Here are five science-backed ways to manage or reverse gut dysbiosis:
1. Prioritize a Gut-Friendly Diet: A diet rich in diverse plant fibers, fermented foods, and polyphenols supports beneficial bacteria. These foods enhance SCFA production, reduce inflammation, and promote microbial diversity (Koh et al., 2016).
2. Limit Antibiotics and Use Judiciously: Only use antibiotics when prescribed. After antibiotic use, prioritize probiotics and prebiotics to repopulate the gut (Langdon et al., 2016). Consider using spore probiotics.
3. Manage Stress Effectively: Chronic stress alters gut barrier function and microbiota composition. Incorporate mindfulness, yoga, journaling, or therapy to balance the gut-brain axis (Chong et al., 2019).
4. Consider Probiotics and Synbiotics: Probiotics are live microorganisms that confer health benefits. Synbiotics combine probiotics and prebiotics. Evidence supports their role in improving gut health (Ouwehand et al., 2002).
5. Seek Professional Guidance: If symptoms persist, consult a healthcare provider. Diagnostic tools like stool tests, SIBO breath tests, and microbiome analysis can uncover underlying causes and guide treatment.
Final Thoughts on Gut Dysbiosis and Human Health
As awareness grows, the importance of addressing gut dysbiosis and human health will become central to preventative medicine and holistic care. Your microbiome plays a foundational role in nearly every bodily system. By taking steps to support a healthy gut—through nutrition, stress management, and professional guidance—you can transform your overall health and longevity.
References
Bordenstein, S. R., & Theis, K. R. (2015). Host biology in light of the microbiome: Ten principles of holobionts and hologenomes. PLoS Biology, 13(8), e1002>
Chong, J., et al. (2019). The microbiota-gut-brain axis: Roles in neurodegenerative and psychiatric disorders. Journal of Physiology, 597(17), 489>
Cryan, J. F., et al. (2019). The microbiota-gut-brain axis. Physiological Reviews, 99(4), 1877–2013.
Dahl, C., et al. (2020). The skin microbiome and its associations with skin health, disease, and immunity. Clinical Microbiology Reviews, 33(4), e00>
David, L. A., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), 559–563.
Dominguez-Bello, M. G., et al. (2010). Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. PNAS, 107(26), 11971–11975.
Foster, J. A., et al. (2017). Stress and the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress, 7, 124–136.
Garrett, W. S. (2015). Cancer and the microbiota. Science, 348(6230), 80–86.
Gupta, S., et al. (2020). Gut microbiota and its role in female infertility. European Journal of Obstetrics & Gynecology and Reproductive Biology, 253, 72–78.
Jernberg, C., et al. (2010). Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology, 156(11), 3216–3223.
Koh, A., et al. (2016). From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell, 165(6), 1332–1345.
Kozik, A. J., et al. (2019). Environmental toxicants and the gut microbiome. Current Environmental Health Reports, 6(1), 1–12.
Langdon, A., et al. (2016). The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Medicine, 8(1), 39.
Le Roy, T., et al. (2013). Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut, 62(12), 1787–1794.
Manichanh, C., et al. (2012). The gut microbiota in IBD. Nature Reviews Gastroenterology & Hepatology, 9(10), 599–608.
Ouwehand, A. C., et al. (2002). Probiotic and other functional microbes: From markets to mechanisms. Current Opinion in Biotechnology, 13(5), 483–487.
Petersen, C., & Round, J. L. (2014). Defining dysbiosis and its influence on host immunity and disease. Cell Microbiology, 16(7), 1024–1033.
Qin, J., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature, 490(7418), 55–60.
Round, J. L., & Mazmanian, S. K. (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology, 9(5), 313–323.
Tang, W. H., et al. (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New England Journal of Medicine, 368(17), 1575–1584.
Turnbaugh, P. J., et al. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature, 444(7122), 1027–1031.
Zmora, N., et al. (2019). Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell, 174(6), 1388–1405.